DIABETES MELLITUS – General Characteristics, Pancreas, Classification, Etiopathogenesis, Pathological Changes, Clinical Features, Diagnosis and Treatment

UPDATED 2024
General Characteristics

Diabetes
mellitus is a chronic metabolic disorder characterized by hyperglycemia with or
without glycosuria, resulting from an absolute or relative deficiency of
insulin. This is brought about by an impairment of insulin production or its
release by the beta cells of the islets of Langerhans. More often it is due to
a resistance to the action of insulin either due to a receptor/post receptor defect
or an imbalance between insulin and its counter regulatory hormones. Clinically
diabetes is characterized by a wide spectrum of disorders ranging from asymptomatic
hyperglycemia to abnormalities in the various organs.
PANCREAS

The
endocrine component of the pancreas consists of different types of cells:
α-cells, β-cells, δ-cells and PP cells contained in the islets of Langerhans
which constitute 1% of its weight. There are 100,000 islets in the pancreas, and
each islet contains 1000-3000 cells. Thus altogether there are 100-300 million
β-cells in the pancreas.
Pancreatic
beta cells can store 200 units of insulin and can release 30-50 units of
insulin per day. 95% of cells of the pancreas have exocrine function and 5%
have endocrine function. The beta cells produce insulin, alpha cells produce
glucagon, delta cells produce somatostatin and the PP cells produce pancreatic polypeptide.
CLASSIFICATION
The
Classification suggested by American Diabetes Association (ADA) is called as
the etiological classification of diabetes and has the two main types of
diabetes labeled as type 1 and type 2, along with gestational diabetes mellitus
and the other specific types where a precise etiological factor is identified.
The revised
diagnostic criteria give equal importance to the fasting and 2 hours post
glucose load plasma glucose (2h PG) for the diagnosis of diabetes, thereby eliminating
the need for a routine oral glucose tolerance test (OGTT) for the diagnosis of
diabetes. The cut-off level of fasting plasma glucose (FPG) for diagnosis of
diabetes has been fixed as 126 mg/dl, since this reflects the same degree of
hyperglycemia as a 2hr-PG of 200 mg/dl in terms of susceptibility for the development
of microvascular and macrovascular complications. These criteria are expected
to rationalize and simplify the diagnosis of diabetes and a larger number of
people could be screened due to the simplification of the procedure of doing
only fasting plasma glucose rather than an OGTT.
ETIOPATHOGENESIS
Type 1Diabetes:
Type-1
diabetes mellitus has been classified into type-1A in which cell-mediated autoimmune
attack on the beta cells is more prominent and type-1B in which the mechanism
is less clear. Type1B is less frequent of the two.
Classification of type-1 diabetes
mellitus
1.
Preclinical
a.
Autoantibodies + / OGTT normal.
b.
Autoantibodies + / OGTT abnormal.
c.
Autoantibodies + / fasting hyperglycemia
2. Clinical – with diabetes
1a.
Autoantibodies present (autoimmune)
1b.
Autoantibodies absent (idiopathic)
3. Explosive onset / fulminent onset
4. Rapid onset
5. Late onset (LADA).
ETIOLOGICAL CLASSIFICATION OF
DIABETES MELLITUS
1. Type 1 diabetes (cell destruction, usually leading
to absolute insulin deficiency)
• Immune
mediated
• Idiopathic
2. Type 2 diabetes (may range from predominantly
insulin resistance with relative insulin deficiency to a predominantly secretory
defect with insulin resistance)
3. Other specific types
A. Genetic defects of cell function
• Chromosome
12, HNF-1 (MODY 3)
• Chromosome
7, glucokinase (MODY 2)
• Chromosome
20, HNF-4 (MODY 1)
•
Mitochondrial DNA
• Others.
B. Genetic defects in insulin action
• Type A
insulin resistance
•
Leprechaunism
•
Rabson-Mendenhall syndrome
•
Lipoatrophic diabetes
• Other
C. Diseases of the exocrine pancreas
•
Pancreatitis, Trauma, Pancreatectomy
• Neoplasia
•
Cystic-Fibrosis, Hemochromatosis
•
Fibrocalculous pancreatopathy
• Others
D. Endocrinopathy
•
Acromegaly/Cushing’s syndrome
• Glucagonoma,
pheochromocytoma
•
Hyperthyroidism, somatostatinoma
•
Aldosteronoma
• Others
E. Drug or chemical induced
•
Pentamidine
• Nicotinic
acid
•
Glucocorticoids
• Thyroid
hormone, diazoxide
• Adrenergic
agonists
• Thiazides,
phenytoin
•
Interferons
• Others. Immunosuppressive
drugs, steroids, tarcrolimus, cyclosporin
F. Infections
• Congenital
rubella
•
Cytomegalovirus
• Others
G. Uncommon forms of immune mediated
diabetes
• Stiff man
syndrome
•
Anti-insulin receptor antibodies
• Others
H. Genetic syndromes associated with
diabetes
• Down’s
syndrome, Turner’s syndrome
•
Klinefelter’s syndrome
• Wolfram’s
syndrome, Friedreich’s ataxia
•
Huntington’s chorea
•
Laurence-Moon-Biedl syndrome
• Myotonic
dystrophy, porphyria
•
Prader-Willi syndrome
• Others
4. Gestational diabetes mellitus
Type 2 DM
Type 2 DM
(previously known as NIDDM) is considered as a ‘multifactorial’ or ‘complex’
disease due to the complex interaction between various genetic and environmental
factors in its pathogenesis. Multiple evidence suggests that genetic factors
play a major role in this condition. A genetic predisposition running through families
is evident. Identical twins invariably develop type 2 diabetes when exposed to
the same environmental factors. In genetically predisposed individuals several environmental
factors precipitate the onset of diabetes.
Important
among these are obesity, physical inactivity, repeated pregnancies, infections,
physical or psychological stress and diabetogenic drugs. Birth of large babies weighing
above 4 kg is a strong pointer to the subsequent development of diabetes in the
mother.
Obesity
The current
obesity epidemic due to the modern sedentary life and caloric abundance is a
major factor that predisposes to type 2diabetes. Hence it is invariably seen that
type 2 diabetes is closely related to obesity. Obese subjects show a relative
resistance to the action of insulin due to a reduction in the number of insulin
receptors on the target cells. The full complement of receptors is restored on
shedding the excess weight.
There is an
association between low birth weight in infancy and occurrence of IGT or DM in
young adulthood. Increase in the body mass index (BMI) after the age of 2 years
is also associated with the chance to develop DM.
Physical Inactivity
It seems to
act as a factor favoring the onset of type 2 diabetes. Many of the diabetics
are physically inactive. Physical exercise improves their exercise tolerance.
Role of
Insulin antagonists: Glucose metabolism is delicately balanced by the coordinated
effects of insulin antagonist hormones like glucagon, cortisol, catecholamines and
growth hormone. Several other hormones also take part in the metabolism of
carbohydrates. Imbalance of this hormonal profile results in carbohydrate intolerance.
Other
antagonists to insulin: Antibodies to insulin may develop in individuals who
are on treatment with insulins especially the animal insulins. The antibodies
inactivate endogenous as well as administered insulin. Such patients show
progressive insulin resistance. Fatty acids which compete with carbohydrate for
metabolism in muscle lead to insulin resistance. In hyperlipidemia insulin
dependent carbohydrate metabolism suffers and a relative insulin resistance
develops.
Thus it
would seem that persons are predisposed to develop type 2 diabetes by
genetically. However lifestyle factors will determine the onset, age of onset,
severity of the metabolic defect and further course.
PATHOLOGICAL CHANGES
Pancreas: In
type 1 diabetes the beta cells of the islets of Langerhans show reduction in
number, degranulation and hyalinization. In recent onset type 1 DM lymphocytic infiltration
of the islets occurs and this may be caused by viral infection. Inflammation is
seen particularly around the beta cells only and not around the other types of
cells.
In type 2 DM
during the early phase the beta cells are normal in number or only slightly
reduced. The beta cells lose their sensitivity to the hyperglycemic stimulus
for releasing insulin. As a result insulin secretion loses its smooth and fine
relationship with glucose level. It tends to be erratic. In the early stages of
evolution of type 2 DM—the reduction in the sensitivity of the receptors is compensated
by overproduction of insulin and accompanying hyperinsulinemia. Frank diabetes
results when beta cells starts failing and insulin production comes down.
Insulin
resistance in muscle develops early in persons who would develop type 2
diabetes later. Beta cell function starts deteriorating about 10 years before
the onset of DM, by which time the beta cell function has fallen to 30% or
less. Acanthosis nigricans is a cutaneous marker of hyperinsulinemia. Both
impaired fasting glucose (1FG) and
impaired
glucose tolerance (IGT) lead to type 2 diabetes in a variable proportion of patients.
Several
factors account for the frequency and time of onset of complications in
diabetes. These include theabnormalities of glucose levels, genetic factors,
smoking, obesity, hypertension, hyperlipidemia and others.
Vascular Changes
Diabetics show
a predisposition to develop vascular lesions affecting both small and large
blood vessels. In microangiopathy, there is specific involvement of the small
blood vessels. Venules, capillaries and arterioles are affected in this
process. There is deposition of PAS (periodic acid Schiff) positive material in
the capillary basement membrane. Glycosylation of several proteins in the
vessel wall results in increased permeability. The basement membrane is
thickened. Ultimately there is vascular occlusion.
Microangiopathy
is most marked in type 1, developing early in life but also occurs in type 2.
Various factors like endothelial damage, increased plasma viscosity,
erythrocyte aggregation, reduced red cell deformability and increased platelet
adhesion lead to microangiopathy. The problem is more complex and the entire
process is still not fully understood. Microangiopathy affects several organ
systems. The main lesions are seen in the retina; kidneys, peripheral nerves and
heart giving rise to diabetic retinopathy, nephropathy, many forms of diabetic
neuropathy and cardiomyopathy.
Macroangiopathy
The diabetic
is prone to develop occlusive vascular disease in medium sized arteries such as
the coronary, cerebral and peripheral limb vessels. The process is one of atheroma
which sets in at younger ages and is more extensive than that occurring in non
diabetics. These lesions lead to increased risk of ischemic heart disease, cerebrovascular
accidents and ischemia to the limbs with intermittent claudication and peripheral
gangrene. Macroangiopathy largely accounts for the steep rise in mortality in
middle-aged diabetics.
Retinopathy
Diabetes
mellitus produces a classical retinopathy. A specific change occurs in the
vessels leading to loss of mural cells (pericytes) and the formation of micro aneurysms.
The occurence of retinopathy is related more to the duration of the disease
than to the severity. Once initiated the fundus changes are usually
progressive. The early changes are venous dilation and the appearance of small dot
like micro aneurysms in the perimacular area.
Arterial
blood is shunted and this leads to ischemia of the retina. Increased vascular
permeability accounts for the formation of exudates. In the next stage dot and
blot hemorrhages predominate. Large subhyaloid hemorrhages and vitreous
hemorrhages may develop and vision is seriously impaired. Such hemorrhages are
due to rupture of newly formed blood vessels. As these hemorrhages are absorbed,
organization by fibrous tissue results and multiple bands of retinitis
proliferans develop. These lead to permanent visual impairment. The fibrous
bands may contract giving rise to retinal detachment. Leaking vessels in the
retina can be demonstrated by fluorescein angiography.
Retinopathy
is usually associated with advanced nephropathy. Sometimes in diabetic
ketoacidosis with severe hyperlipidemia the fat gives a milky white appearance
to the retinal arteries called “lipemia retinalis”
Renal Lesions
These are
commonly seen in subjects who have had diabetes for over 15-20 years. Vascular
changes include (i) arteriosclerosis of the renal artery, (ii) sclerosis of the
arterioles and (iii) glomerulosclerosis. Glomerulosclerosis may be nodular
(Kimmelstiel-wilson lesion) or diffuse. There is accumulation of PAS positive eosinophilic
material within the mesangium. There is thickening of the glomerular capillary
basement membrane. The establishment of glomerulosclerosis is indicated by the
presence of proteinuria. Further damage to the glomeruli results in the
development of chronic renal failure.
Distant
stages can be defined in the evolution of diabetic nephropathy. In the initial
stages, asymptomatic microalbuminuria in which up to 200 mcg/minute of albumin
may be lost in the urine. Normal subjects do not lose more than 20 mcg/min or
300 mg of protein in 24 hours. Microalbuminuria is not detectable by the
ordinary laboratory tests. In type 1 DM there is elevation of systotic BP
during sleep preceding micro albuminuria. This rise in BP is an important
contributory factor in the development of structural changes in the kidneys. It
is absolutely necessary to control blood pressure also along with blood sugar
to prevent deterioration.
In the early
stage the kidneys are enlarged, more vascular and the glomerular filtration
rate (GFR) is increased. In the second stage, there is microalbuminuria and in
third stage the proteinuria is more pronounced and easily detectable by routine
tests. Loss of 3.5g or more of protein in 24 hours may lead to the development
of nephrotic syndrome. Hypertension develops during this stage. In the fourth
stage further structural changes develop and the glomerular filtration rate
comes down with gradual increase in the blood levels of metabolic waste
products such as creatinine and urea. The fifth stage is one of gross reduction
of glomerular filtration and overt renal failure with azotemia, severe
hypertension and complications such as cardiac failure and end stage renal failure.
Autonomic neuropathy may lead to functional obstruction of the bladder,
retention with over flow, urinary infection and further deterioration of renal functions.
Another system of classification is based on creatinine clearance.
The diabetic
patient is predisposed to develop urinary infection and therefore acute and chronic
pyelonephritis are very common. Fulminant urinary infection leads to ischemic
necrosis of the renal papillae. This presents as acute anuric renal failure.
Fleshy masses may be passed in the urine. These are the necrosed papillae and
the condition is called papillitis necroticans ulcerans.
Emphysematouspyelonephritis is another serious complication.
Peripheral Nerves
In the
myelinated nerve fibers, axonal atrophy was considered to be the primary
lesion, secondary to ineffective axonal transport. Axoglial dysfunction, and abnormalities
of paranodal connections between the terminal myelin loops and the axonal
membrane have also been described. This could explain the reduction in nerve conduction
velocity. This improves with therapeutic inhibition of aldose reductase. More
recent studies, however provide evidence for the presence of demyelination and
hence Schwann’s cell involvement is the primary lesion. As the myelinated
fibers degenerate, there is an attempt to regenerate, which manifests in the form
of regeneration clusters. With progress of the neuropathy the density of the
regeneration clusters also comes down. Structural abnormalities have also been
found in the vessels supplying the nerve fibers. The epineural vessels show
arteriolar attenuation, venous distension, arteriovenous shunting and new
vessel formation along with intimal hyperplasia and hypertrophy, denervation
and reduction in neuropeptide expression. The perineural vessels also
demonstrate basement membrane thickening and endothelial cell hypertrophy and
hyperplasia. There is also a reduction in capillary density and occurrence of pericyte
loss with reduction of endoneural oxygen tension and blood flow to the nerves.
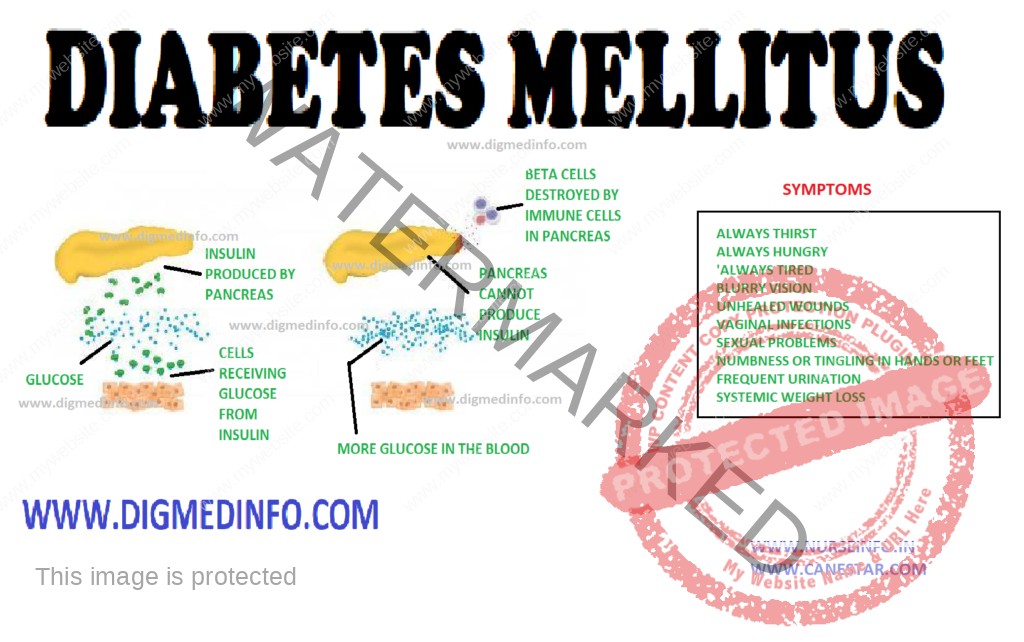
CLINICAL FEATURES
The clinical
manifestations of diabetes are protean. Though the symptoms are similar in both
types of diabetes, in type 1 they develop acutely whereas in the majority of
the type 2 the onset is insidious. Type 1 patients are usually below the age of
30, thin and emaciated and unless promptly treated with insulin, they would
develop ketoacidosis.
Due to the
high prevalence of obesity, type 2 diabetes is occurring at earlier ages as a
global phenomenon, especially in developed countries.
Type 2
patients are generally above the age of 30, obese, usually asymptomatic and may
present directly with the vascular complications of diabetes. Around 50% of the
cases present with the classical symptoms of polyuria, polyphagia and weight
loss. These symptoms can be directly correlated with hyperglycemia and glycosuria.
Other clinical presentations which warrant full investigation to exclude
diabetes are (i) non-healing ulcers (ii) recurrent respiratory or urinary tract
infections (iii) Rapid changes in refraction of the eyes (iv) steady and unexplained
rapid weight loss (v) increased tendency for fungal infections like moniliasis,
balanoposthitis and vulvitis; (vi) unexplained peripheral neuropathy (vii)
premature onset of ischemic heart disease, stroke or vascular occlusions (viii)
history of overweight babies and recurrent fetal loss in women (ix) premature
cataract often below the age of 50 years and retinopathy (x) impotence in
males, and (xi) any vague ill-health. In some cases, diabetics may present to
the doctor for the first time with any of the major emergencies, without any apparent
illness previously.
DIAGNOSIS
Diabetics
with classic symptoms can be diagnosed clinically, but since many cases may be
asymptomatic, diabetes should be suspected even in the absence of symptoms. The
clinical symptoms and the biochemical alterations do not go hand in hand in
many cases. Diabetes being mainly a biochemical disease with several different but
inter-related biochemical and molecular abnormalities, should always be
diagnosed and managed with biochemical monitoring along with clinical
examination.
Fasting
plasma glucose levels above 126 mg/dL (6.7 mmol/L) or postprandial plasma
glucose levels above 200 mg/dL are diagnostic. It is always better to do
bothestimations to confirm the diagnosis.
Estimation
of FBS and PPBS has become mandatory investigations in all health check up
examinations.
Urine tests: These tests can be used for initial
screening and follow-up of cases under treatment. Urinary glucose does not
always directly reflect the blood glucose level. The value of urine examination
cannot be underestimated since protenuria, ketonuria and the microscopic
abnormalities can be detected only by examining the urine.
Glucose in
urine is tested by the Benedict’s test and Clinitest (Chemtab), which detect
reducing substances non-specifically. While glucose is by far the most common reducing
substance in urine, the possibility of other reducing substance should be kept
in mind and the enzyme methods (employing glucose oxidase) which are specific for
glucose (Clinistix, Diastix) should be employed. If the Benedict’s test is
positive and the glucose oxidase is negative, the presence of other reducing substances
such as ascorbic acid, aspirin and lactose should be suspected.
Blood glucose estimation: Various methods are employed to
estimate the blood glucose. The methods using copper reduction (Folin-Wu or
Nelson Somogyi) also detect the reducing substances like uric acid, creatinine
and hence their values are 20-30 mg/dL higher than those obtained by the
glucose oxidsae method which gives the true glucose values. Highly accurate and
rapid (1-2 min) devices are now available based on immobilized glucose oxidase
electrodes. Hexokinase and glucose dehydrogenase methods are used for
reference. Blood sugar estimations are mandatory for confirming the diagnosis of
diabetes. Both fasting and postprandial values should be estimated. In mild
diabetes the fasting blood sugar values may be below 126 mg /dL and therefore
the diagnosis is likely to be missed if only the fasting blood sugar is
estimated.
Glucose tolerance test (GTT): The oral glucose tolerance test
(OGTT) is mainly used for diagnosis of diabetes when blood glucose levels are
equivocal, during pregnancy, or in an epidemiological setting to screen for
diabetes and impaired glucose tolerance.
The OGTT
should be administered in the morning after at least 3 days of unrestricted
diet (greater than 150 g of carbohydrate daily) and usual physical activity.
The test should be preceded by an overnight fast of 8-14 h. during which period
only water may be drunk. Smoking is not permitted during the test. The presence
of factors such as medications, inactivity and infection that influence interpretation
of the results of the test must be recorded.
After
collection of the fasting blood sample, the subject should drink 75 g of
anhydrous glucose dissolved in 250-300 mLof water over the course of 5 minutes.
For children, the test load should be 1.75g of glucose per kg of body weight,
up to a maximum of 75g of glucose. Blood samples should be once again collected
2 hr after the test load.
If there is
delay in estimation of glucose the blood samples should be collected in a tube
containing sodium fluoride (6 mg/mL of whole blood) and immediately centrifuged
to separate the plasma. In subjects having symptoms of diabetes, a single
fasting value above 126 mg/dL or a 2-hour blood glucose value above 200 mg/dL
after 75 g of glucose oral may be taken to be diagnostic. In asymptomatic
subjects at least two abnormal blood glucose values should be insisted upon to
confirm the clinical diagnosis.
TREATMENT
The aim of treatment is to achieve normal blood glucose levels throughout day and night, to alleviate symptoms and to prevent complications. The four pillars of diabetic management are diet, exercise, drugs and patient education, backed up by regular monitoring of glycemic control and early detection and treatment of complications.
Discover more from Bibliobazar Digi Books
Subscribe to get the latest posts sent to your email.




![First Aid (Quick Study Health) PDF Free Download [Direct Link]](https://bazarbiblio.com/wp-content/uploads/2024/02/First-Aid-Quick-Study-Health-PDF.jpg)